Calculate what you would make at Buffer using this online calculator based on the most recent Buffer salary formula. Buffer Zone Calculator. Game slots casino. This tool, developed by EPA, is specific to each fumigant product and is based on the look-up tables on the product labels. In addition to calculating buffer zone distances, the calculator can also be used to quickly calculate buffer zone reductions through the use of credits and modifications to application parameters. Chemical/biochemical pocket companion designed by the editors of Current Protocols. Choose from a list of all of the commonly used biological buffer systems, enter your desired volume, pH, and buffer concentration, as well as the concentrations of your stock solutions of the conjugate acid and base, and the calculator will inform you of the exact volumes of these stock solutions to mix in. Acetate Buffer Calculator. A solution which has a stable pH is termed as a buffer solution. It is a mixture of weak acid and the base formed by the weak acid when it donates one proton. A buffer solution formed by the mixture of acetic acid and sodium acetate (base formed by acetic acid) is acetate buffer. Buffer is a fully remote team, spread across the whole planet. We have Bufferoos in 15 countries, 11 timezones and 42 cities (and counting!). As a member of our team, you will be invited to work wherever you're happiest and most productive.
Instructions
Type the desired pH into the first cell, and type the intended buffer strength (in millimoles per liter) in the second cell. Press the calculate button, and the approximate concentrations of monosodium phosphate, monohydrate and disodium phosphate, heptahydrate will be displayed.Buffer Calculator Sigma
How the calculation works
Using Phosphate as the example: Before calculations can begin, the pKa's of phosphoric acid must be adjusted. The ions interfere with the idealized numbers provided by the pKa's at the link. To adjust for these, we apply the Debye-Huckel equation using A at 0.509 and calculating I by assuming that half of the buffer strength is divided between the conjugate acid and base of each stage of the buffer. So, for a buffer strength of 0.1 M, the program assumes that [H3PO4] = 0.05 M and [H2PO4Citrate Buffer Calculator
-] = 0.05 M to calculate the pKa change for H3PO4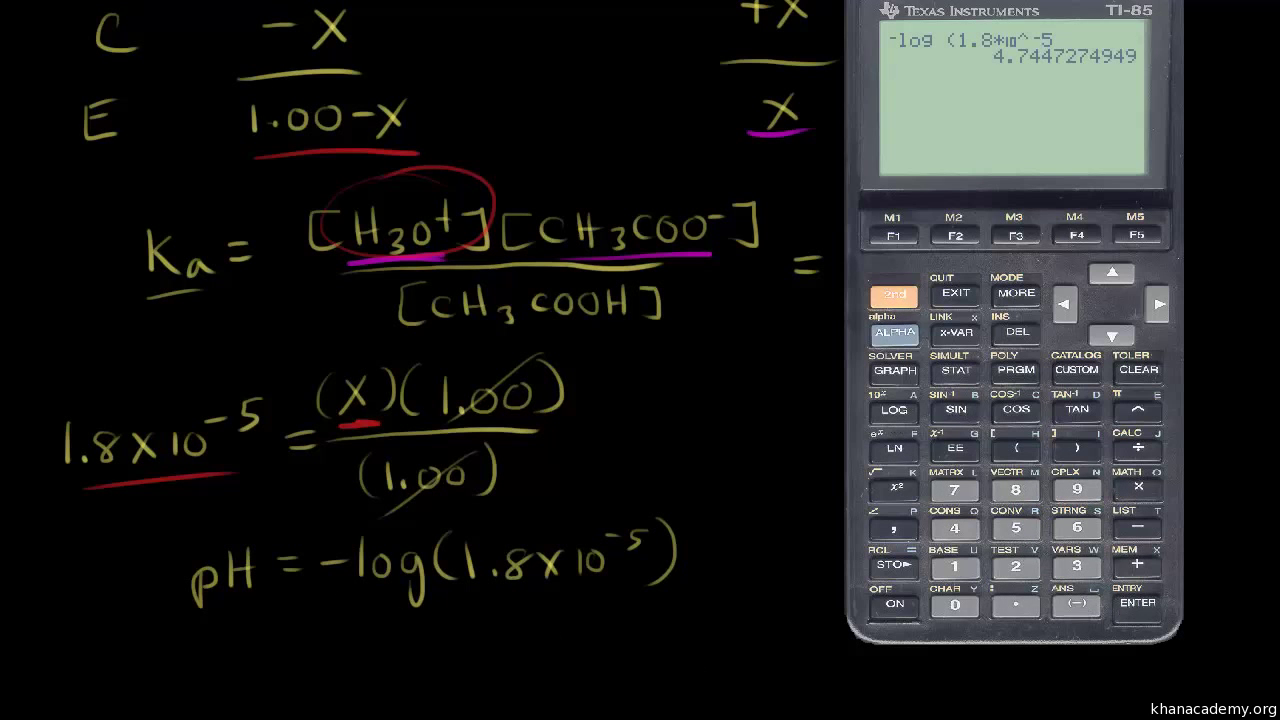

Using the three adjusted pKa's of phosphoric acid and the pH, the ratios of each of the phosphoric pairs are calculated. For example, the first ionization is given by: H3PO4 --> H+ + H2PO
K1 = [H+][H2PO4-] / [H3PO4],
or [H2PO4-] / [H3PO4] = K1 / [H+]
[Na2HPO4] = [Na] - Buffer StrengthThe pKa's for phosphoric acid are 2.15, 7.20, and 12.38 at 25°C. Buffers made with the above salts work best in the pH range 6-10. What buffer strength to use? Too low will give a weak, drifting buffer (low buffer capacity), while too much may negatively affect other desired properties, such as taste.
[NaH2PO4] = Buffer Strength - [Na2HPO4]
Version 2, Dec 2, 2011. Scott Calabrese Barton (source code)
Version 1.1, Jan 19, 2000. Scott Calabrese Barton
Version No. 1, December 31, 2000. Jeffrey Clymer
Index of other pages by Jeffrey Clymer Online casino games for real money canada.
Using the three adjusted pKa's of phosphoric acid and the pH, the ratios of each of the phosphoric pairs are calculated. For example, the first ionization is given by: H3PO4 --> H+ + H2PO4-
K1 = [H+][H2PO4-] / [H3PO4],
or [H2PO4-] / [H3PO4] = K1 / [H+]
[Na2HPO4] = [Na] - Buffer StrengthThe pKa's for phosphoric acid are 2.15, 7.20, and 12.38 at 25°C. Buffers made with the above salts work best in the pH range 6-10. What buffer strength to use? Too low will give a weak, drifting buffer (low buffer capacity), while too much may negatively affect other desired properties, such as taste.
[NaH2PO4] = Buffer Strength - [Na2HPO4]
Version 2, Dec 2, 2011. Scott Calabrese Barton (source code)
Version 1.1, Jan 19, 2000. Scott Calabrese Barton
Version No. 1, December 31, 2000. Jeffrey Clymer
Index of other pages by Jeffrey Clymer Online casino games for real money canada.
- Makes corrections for the temperature at which the buffer will be used
- Makes Debye-Huckel corrections for the effect of ionic strength on pKa
- Describes two ways (titration or by accurate weight) for preparation of the recipe

